Interactions
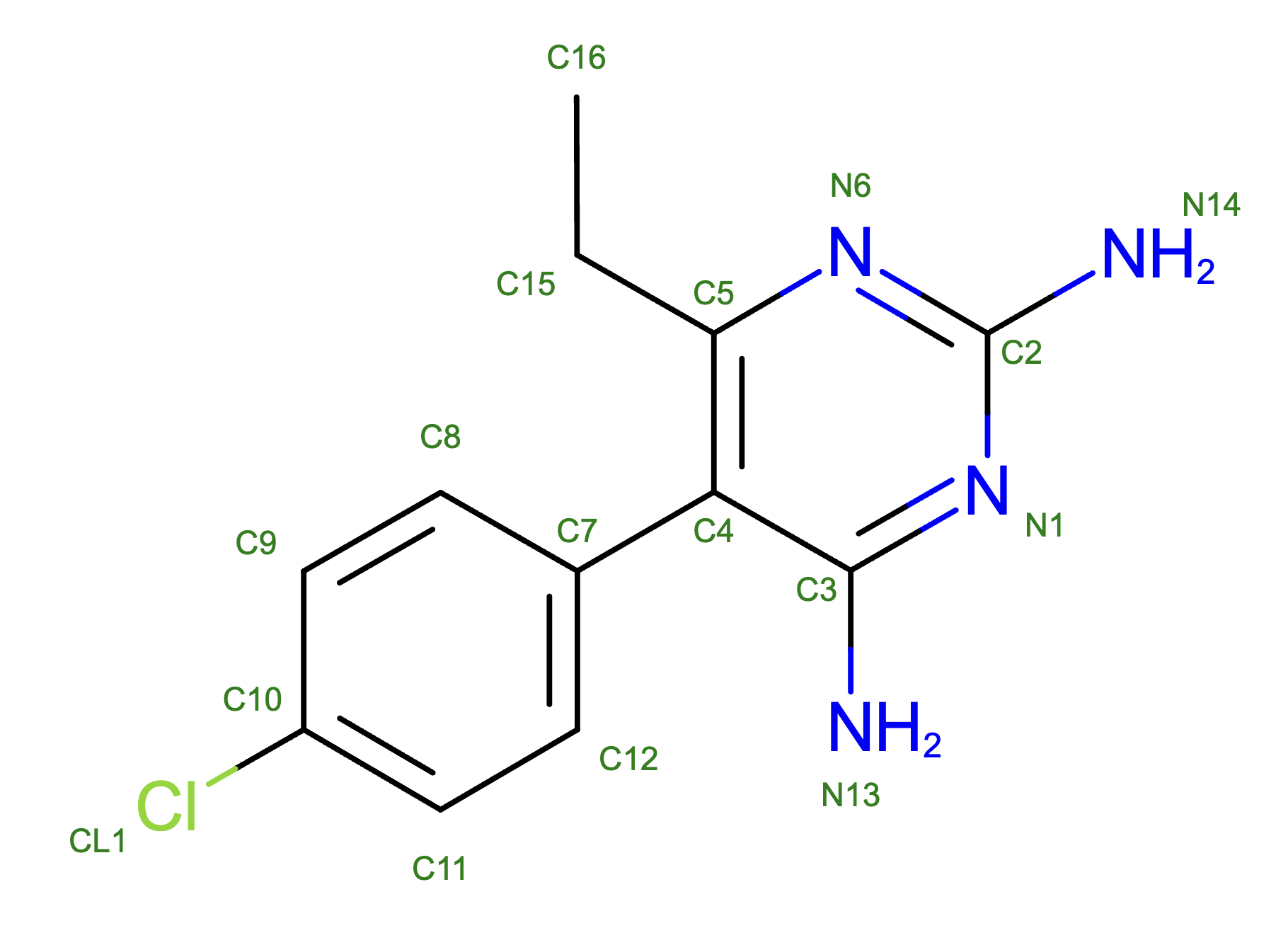
Pyrimethamine, referred to as ligand CP6 on the Protein Data Bank [1], contains 1 chlorine group, which creates a partial negative charge on the C10 carbon, which is part of the molecule’s benzene ring. On the other end of the molecule is a pyrimidine ring, which consists of: 2 partially positive charged amine groups on C2 and C3, an ethyl group on C5, and a connection to the C7 carbon of the benzene ring on C4 . The overall formal charge of the molecule is 0. It interacts with the amino acids serine, threonine, isoleucine, asparagine, and leucine. Serine and threonine have the highest binding affinities with CP6, as both are polar side chains with a hydroxyl group. They are followed by isoleucine with a hydrophobic side chain and asparagine with a carbonyl and amine group. Finally, leucine is the amino acid with the lowest known affinity with CP6. Other amino acids also bind to CP6, but their affinities are unknown.
6AOG: Bi-functional dihydrofolate reductase-thymidylate synthase

2 CP6 molecules can bind to chains F and J of the protein, and in both cases the benzene ring is attracted to a pyridine R-group in both chains. The 2 amine groups on CP6 also form hydrogen bonds with oxygen on both chains of 6AOG (1 by N13 and 2 by N14). [2]
1J3J: Double Mutant Plasmodium falciparum dihydrofolate reductase-thymidylate synthase

CP6s Cl group are attracted to an oxygen from 1J3J’s R-groups on both chain E and G. On chain E, there is an attraction between CP6 and 1J3J’s benzene rings, as well as interactions between the 2 amine groups and the surrounding R-groups (2 by N13, 3 by N14). Whereas on chain G, the pyrimidine group is attracted to a benzene ring on 1J3J, and both amine groups are each attracted to 3 oxygen molecules on the surrounding R-groups. [3]
2BL9: Plasmodium Vivax dihydrofolate reductase

CP6 binds on chain C alone, where the Cl group is attracted to an oxygen on the surrounding protein. Furthermore, the 2 amine groups on the pyrimidine ring form 6 attractions with chain C’s R-groups (2 by N13, 4 by N14). [4]
1U72: Dihydrofolate reductase

MANORAA's Ligand Interaction Tool
Below is an interactive window to model the ligand interactions and binding of the chlorine group in pyrimethamine, hosted by MANORAA.ai. Please scroll down and explore the ligand interactions by selecting the relevant complex and amino acid.
References
- https://www.rcsb.org/ligand/CP6
- https://www.rcsb.org/structure/6aog
- https://www.rcsb.org/structure/1j3j
- https://www.rcsb.org/structure/2BL9
- https://www.rcsb.org/structure/1u72