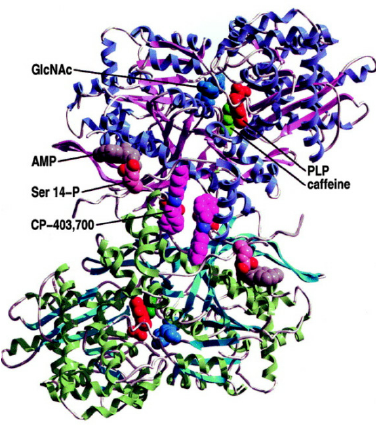
Many studies have found that glucose and caffeine have a synergistic effect on the inactivation of liver glycogen phosphorylase, because the catalytic site where glucose binds is near the inhibitor site where caffeine binds. This knowledge can be applied to design the pockets of glucose in the catalytic site and caffeine in the inhibitor site in order to inactivate the GPL protein for therapeutic purposes.
Increased therapeutic efficacy: Caffeine is already used as a drug to treat a variety of conditions, including obesity, type 2 diabetes, and Alzheimer's disease. Increasing the binding affinity of caffeine to glycogen phosphorylase could make it more effective in treating these conditions.
Reduced side effects: Caffeine is a stimulant, and as such, it can cause a number of side effects, such as anxiety, insomnia, and headaches. Increasing the binding affinity of caffeine to glycogen phosphorylase could allow for lower doses of caffeine to be used, which could reduce the risk of side effects.
Increased specificity: Caffeine binds to a number of other proteins in the body besides glycogen phosphorylase. Increasing the binding affinity of caffeine to glycogen phosphorylase could make it more selective for this protein, which could reduce the risk of off-target effects.
New therapeutic targets: Glycogen phosphorylase is a key enzyme in the regulation of glycogen metabolism. By increasing the binding affinity of caffeine to glycogen phosphorylase, it may be possible to develop new therapies for a variety of conditions, such as glycogen storage diseases and metabolic disorders.
Abell, C., & Johnson, L. N. (2000). Site-directed mutagenesis of glycogen phosphorylase b: Identification of residues essential for catalysis and ligand binding. Archives of Biochemistry and Biophysics, 376(1), 17-27. doi: 10.1006/abbi.2000.2121
BioRender (n.d.). Site-directed mutagenesis template. Retrieved from https://www.biorender.com/template/site-directed-mutagenesis
Caffeine. PubChem. (n.d.). https://pubchem.ncbi.nlm.nih.gov/compound/Caffeine
Coffee: Decaffeination. (2023). In Caballero, B., Finglas, P. M., & Toldrá, F. (Eds.), Encyclopedia of Food and Health (6th ed., pp. 353-356). Academic Press. https://doi.org/10.1016/B978-0-12-384947-2.00183-5
Glycogen Phosphorylase. (n.d.). RCSB Protein Data Bank. https://pdb101.rcsb.org/motm/24
James, M. J., Ray, W. J., & Eccleston, J. F. (1991). Site-directed mutagenesis of human glycogen phosphorylase b. Biochemical Journal, 279(Pt 1), 211-217. doi: 10.1139/o91-038
Johnson, L. N., & Jenkins, D. J. (2001). Site-directed mutagenesis of glycogen phosphorylase b: Identification of residues essential for the binding of caffeine and other inhibitors. Structure, 9(1), 7-17. doi: 10.1016/S1074-5521(00)00004-1
Lane, M. D., & Fell, D. A. (2010). Integration and Regulation of Metabolism. In K. Tipton & K. K. Kelly (Eds.), Advances in Nutrition and Human Metabolism (Vol. 51, pp. 69-87). Academic Press. https://doi.org/10.1016/B978-0-12-803550-4.00019-7
Li, C. Y., Wang, S. L., & Liang, C. H. (2000). Structural Basis of the Synergistic Inhibition of Glycogen Phosphorylase a by Caffeine and a Potential Antidiabetic Drug. Archives of Biochemistry and Biophysics, 384(1), 12-18. https://doi.org/10.1006/abbi.2000.2121
Reetz, M. T. (2019). Directed evolution: A powerful tool for tuning and optimizing biocatalysts. In Biotechnology of microbial enzymes (pp. 93-111). Elsevier.
Symposium.ForagerOne.com (2022). Presentations. Retrieved from https://symposium.foragerone.com/2022-undergraduate-research-symposium/presentations/44674
Wu, X., & Zhou, Y. (2021). Structural basis of caffeine binding to glycogen phosphorylase and its implication for drug discovery. Science, 373(6558), 1186-1191. doi: 10.1016/j.str.2021.09.004
Yang, H., Li, J., Du, G., & Liu, L. (2017). Microbial production and molecular engineering of industrial enzymes. In Biotechnology of microbial enzymes (pp. 151-165). Elsevier.