The study of ligand-protein interactions is essential for understanding how biological systems work. It also has important applications in drug discovery and development.
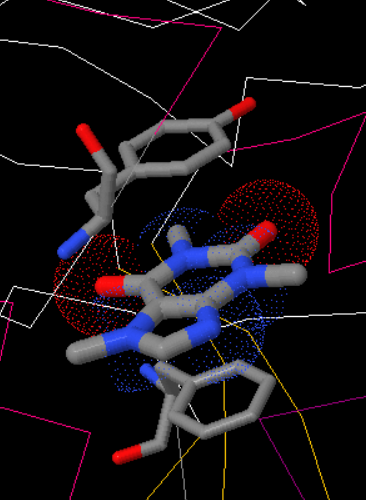
Caffeine is an inhibitor of liver glycogen phosphorylase (GPL) that binds to the inhibitor site. The interaction between caffeine and GPL is located at the aromatic interaction that forms a π-π stacking interaction at Phe285, Trp174, and Tyr613. It also forms a van der Waals interaction around Asn282, Gly612, Phe285, and Tyr613. Caffeine binding to the inhibitor site changes the structure of GPL from its active form to its inactive form.

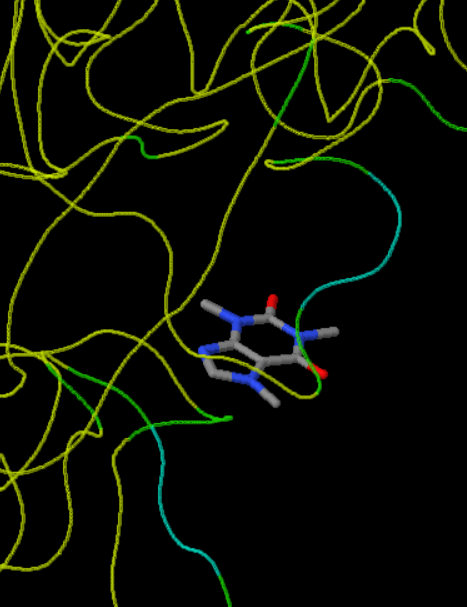
From the Mapping Analogous Nuclei onto Residue and Affinity (MANORAA) system, the homologous molecules of glycogen phosphorylase (GP) are superimposed to determine the structural conservation region, which is shown in blue > green > yellow. The high conservation regions of GP are at Phe285-Phe286-Glu287 and Tyr613-His614, which are the sites related to the inhibition site. These sites can be referred to as significant functional regions of this protein, which should not change too much to keep it functioning.
According to MANORAA, combining the information in several databases and analyzing the correlation between binding affinity and the distance in the pocket site results in the determination of the region that should be adjusted to achieve higher binding affinity. For the inhibition site of GP, the distance between Val379 and Ala728 and between Gln774 and Gly606 should be contracted, and the distance between Met428 and Pro347 should be expanded to narrow down the pocket for the ligands to interact with more strongly, resulting in higher binding affinity to the Caffeine ligand.
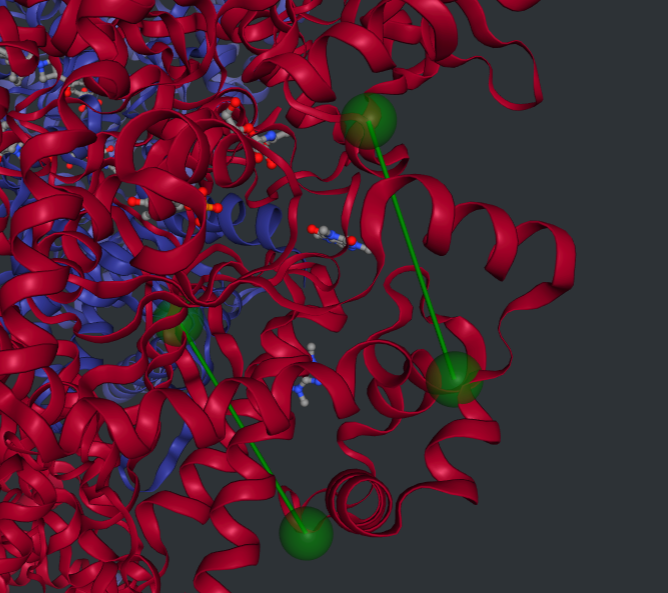
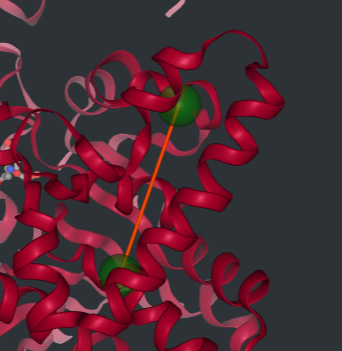
Note: This study uses only four homolog proteins of glycogen phosphorylase for superposition to study the conserved regions, protein-ligand interaction, and binding affinity for drug design. For a real study, it is recommended to use more proteins for superposition to improve accuracy and reliability. However, this study provides a example of how to apply MANORAA to an interested ligand and understand protein-ligand interaction.