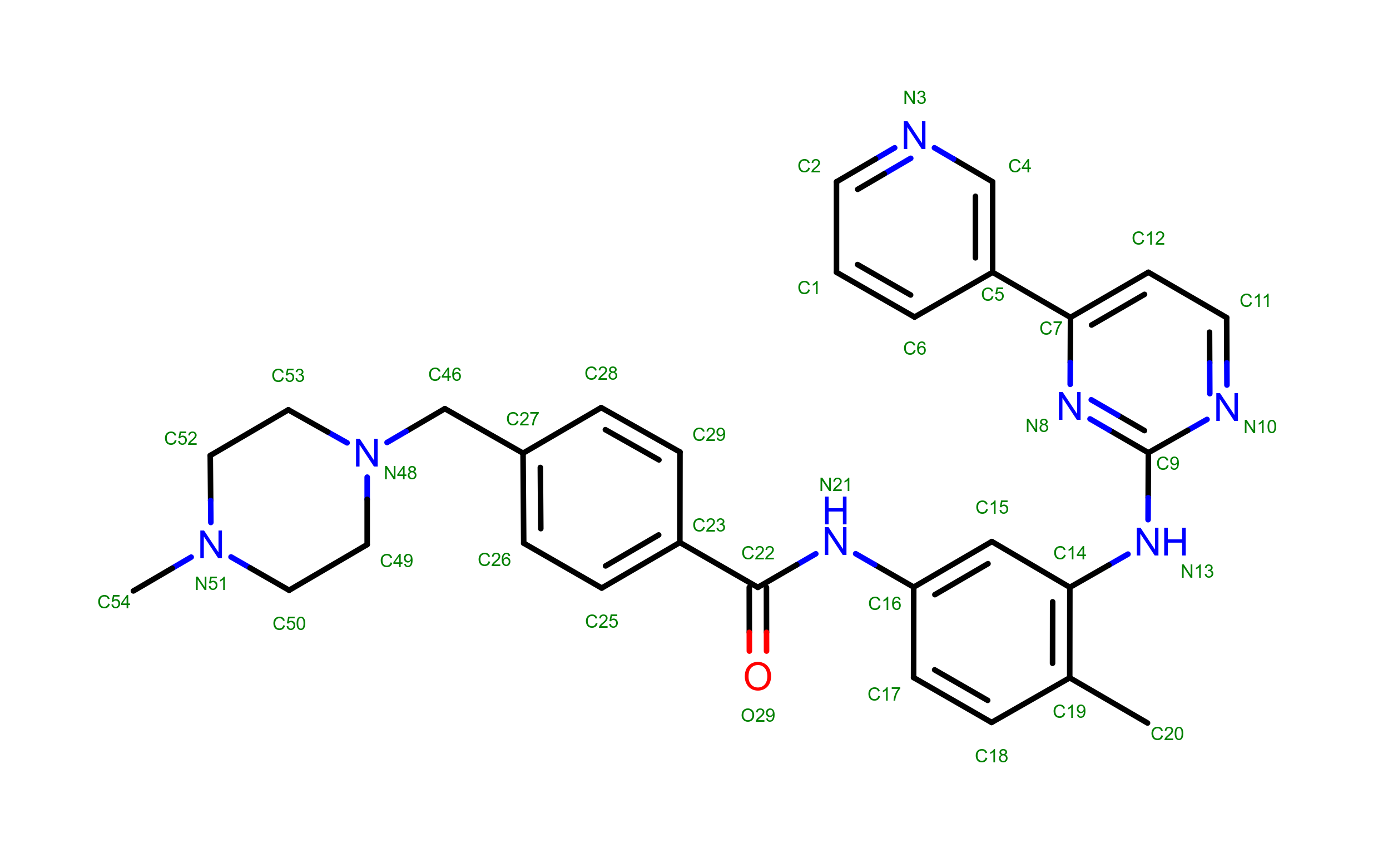
Molecular Diagram
The Significance of Structural Studies
Imatinib is a specific drug targeted at the oncogenic protein BCR-ABL1. It has been designed based on the structural analysis wild type human ABL1 (hABL1) protein. The structural analysis of the drug target target BCR-ABL1 and the conserved residues and atoms with other potential targets can provide insight on the selectivity of the drug.
The drug acts as a competitive inhibitor for the ATP binding site on the tyrosine kinase domain of BCR-ABL1. This inhibites the phosphorylation activity of the fusion protein as ATP can no longer bind to the enzyme to be used as a source of phosphate.
With targeted cancer therapy like imatinib, a high specificity is desirable so that the drug only targets the cancer cells. This minimises the off-target effects and reduces side effects of the therapy. To achieve this, the ligand interaction between the key residues and atoms of the binding pocket is analysed. A high specificity is achieved when the ligand interacts with atoms that are not highly conserved, meaning binding affinity is reduced with binding pockets other than the target.
Conserved Atoms Between Binding Partners
Below is a superposition of numerous complexes associated with imatinib. Regions with some position specific conservation are highlighted in green, and regions with higher conservation are highlighted in blue. It is expected that the conserved regions contain position specific atoms which are key to the structural functionalities of the active site.
However, they do not necessarily indicate the key atoms that interact with imatinib for binding, rather they may demonstrate the lack of such atoms. This is due to the aim of this targeted therapy drug to have the highest binding affinity to its target and lower affinity with any other potential partners to minimise off target effects.